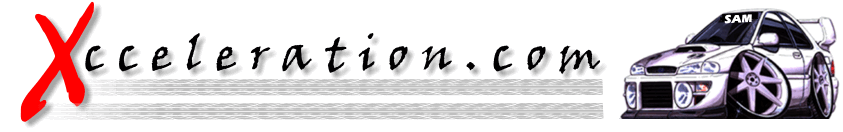|
"What's better, low
compression and more boost or high compression and less boost?"
There
are certainly reasons to try to raise compression ratio, namely when
off-boost performance matters, like on a stree tcar, or when using a
very small displacement motor. But when talking purely about on-boost
power potential, compression just doesn't make any sense.
People
have tested the power effects of raising compression for decades, and
the most optimistic results are about 3% more power with an additional
point of compression (going from 9:1 to 10:1, for example). All
combinations will be limited by detonation at some boost and timing
threshold, regardless of the fuel used. The decrease in compression
allows you to run more boost, which introduces more oxygen into the
cylinder. Raising the boost from 14psi to 15psi (just a 1psi increase)
adds an additional 3.4% of oxygen. So right there, you are already past
the break-even mark of losing a point of compression. And obviously,
lowering the compression a full point allows you to run much more than
1 additional psi of boost. In other words, you always pick up more
power by adding boost and lowering compression, because power potential
is based primarily on your ability to burn fuel, and that is directly
proportional to the amount of oxygen that you have in the cylinder.
Raising compression doesn't change the amount of oxygen/fuel in the
cylinder; it just squeezes it a bit more.
So
the big question becomes, how much boost do we gain for X amount of
compression? The best method we have found is to calculate the
effective compression ratio (ECR) with boost. The problem is that most
people use an incorrect formula that says that 14.7psi of boost on a
8.5:1 motor is a 17:1 ECR. So how in the world do people get away with
this combination on pump gas? You can't even idle down the street on
pump gas on a true 17:1 compression motor. Here's the real formula to
use:
sqrt((boost+14.7)/14.7) * CR = ECR
sqrt = square root
boost = psi of boost
CR = static compression ratio of the
motor
ECR = effective compression ratio
So
our above example gives an ECR of 12.0:1. This makes perfect sense,
because 12:1 is considered to be the max safe limit with aluminum heads
on pump gas, and 15psi is about as much boost as you can safely run
before you at least start losing a significant amount of timing to
knock. Of course every motor is different, and no formula is going to
be perfect for all combinations, but this one is vastly better than the
standard formula (which leaves out the square root).
So
now we can target a certain ECR, say 12.0:1. We see that at 8.5:1 CR we
can run 14.7psi of boost. But at 7.5:1 we can run 23psi of boost (and
still maintain the 12.0:1 ECR). We only gave up 1 point of compression
(3% max power) and yet we gained 28% more oxygen (28% more power
potential). Suddenly it's quite obvious why top fuel is running 5:1
compression, that's where all the power is!!
8.5:1
turns out to be a real good all around number for on and off boost
performance. Many "performance" NA motors are only 9.0:1 so we're not
far off of that, and yet we're low enough to run 30+ psi without
problems (provided that a proper fuel is used).
Example: "I've got a 500+ CID motor and
I'm looking to make 900hp. Can I use a GT42, I've heard they can make
900hp?"
Nope!
There's nothing wrong with the GT42, it will definitely make 900hp,
just not in this scenario. Here's why: 900hp represents a fairly
constant amount of air/fuel mixture, regardless of whether it's being
made by a small motor at high boost (e.g. 183ci at 32psi) or a large
motor at low boost (e.g. 502ci at 10psi).
The
first problem is that most compressors are only able to reach their
maximum airflow when they are running at high boost levels. For
example, a GT42 is able to flow about 94lbs/min of air at 32psi of
boost, but it can only flow around 64lbs/min of air at 10psi. Often
people are quick to assume that high boost means high heat and
therefore decreased efficiency, but in reality, it takes higher boost
levels to put most turbos into their "sweet spot". In this particular
example, the turbo is capable of almost 50% more HP at high boost
levels than it is at low boost levels.
The
other problem is related to backpressure. If the exhaust system
(headers, turbine, downpipe, etc.) is the same between both motors, the
backpressure will be roughly the same. Let's say the backpressure
measures at 48psi between the motor and turbine. The big motor will run
into a bottleneck because there is 48psi in the exhaust and only 10psi
in the intake (a 4.8:1 ratio). This keeps the cylinder from
scavenging/filling fully and therefore limits power. The small motor,
on the other hand, has 32psi of boost (only a 1.5:1 ratio) to push
against the backpressure. Therefore it is able to be much more
efficient under these conditions.
The
bottom line is, as your motor size increases, your boost level will go
down (in order to achieve the same power level). In such a case you
will need to maximize the flow potential of your compressor and
minimize the restriction of your exhaust system (including the turbine)
in order to reach your power goals.
FINAL COMPRESSION RATIO
CHART
This
chart shows the final compression ratio in your engine by combining the
static compression ratio read down the left side and the amount of
boost applied to the engine across the top. Use this chart shown below
as a guideline to determine the proper amount of maximum boost level
for a specific application.
Final
compression ratios above 12.4 to 1 are not recommended for use with
"premium pump gasoline." The higher the final compression ratio the
higher the octane rating of the gasoline must be in order to help
prevent detonation and serious engine damage.
Final Compression Ratio (FCR) = [(Boost/
14.7) +1] x CR
boost = psi of boost
CR = static compression ratio of the
motor
FCR = final compression ratio
Altitude
plays an important role in determining compression ratios. If the
altitude in the area where the vehicle is driven is significantly
higher than sea level then the compression ratios will vary. To
determine the effects of the altitude on a calculated compression ratio
use the following formula:
Correct Compression Ratio = FCR
minus [(altitude/1000) x 0.2]
| Compression |
|
|
|
|
|
|
|
|
|
|
|
| Ratio |
|
|
|
|
Boost (in
pounds per square inch) |
|
|
| |
2
|
4
|
6
|
8
|
10
|
12
|
14
|
16
|
18
|
20
|
22
|
24
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| 6.5:1 |
7.4 |
8.3 |
9.2 |
10.0 |
10.9 |
11.8 |
12.7 |
13.6 |
14.5 |
15.3 |
16.2 |
17.0 |
| 7.0:1 |
8.0 |
8.9 |
9.9 |
10.8 |
11.8 |
12.7 |
13.6 |
14.5 |
15.3 |
16.2 |
17.0 |
17.9 |
| 7.5:1 |
8.5 |
9.5 |
10.6 |
11.6 |
12.6 |
13.6 |
14.6 |
15.7 |
16.7 |
17.8 |
18.6 |
19.5 |
| 8.0:1 |
9.1 |
10.2 |
11.3 |
12.4 |
13.4 |
14.5 |
15.6 |
16.7 |
17.8 |
18.9 |
19.8 |
20.9 |
| 8.5:1 |
9.7 |
10.8 |
12.0 |
13.1 |
14.3 |
15.4 |
16.6 |
17.8 |
18.9 |
19.8 |
20.9 |
21.9 |
| 9.0:1 |
10.2 |
11.4 |
12.7 |
13.9 |
15.1 |
16.3 |
17.6 |
18.8 |
20.0 |
21.2 |
22.4 |
23.6 |
| 9.5:1 |
10.8 |
12.1 |
13.4 |
14.7 |
16.0 |
17.3 |
18.5 |
19.8 |
21.1 |
22.4 |
23.6 |
24.8 |
| 10.0:1 |
11.4 |
12.7 |
14.1 |
15.4 |
16.8 |
18.2 |
19.5 |
20.9 |
22.2 |
23.6 |
24.8 |
26.0 |
| 10.5:1 |
11.9 |
13.4 |
14.8 |
16.2 |
17.6 |
19.1 |
20.5 |
21.9 |
23.4 |
24.8 |
26.2 |
27.6 |
| 11.0:1 |
12.5 |
14.0 |
15.5 |
17.0 |
18.5 |
20.0 |
21.5 |
22.9 |
24.5 |
26.0 |
27.5 |
28.9 |
|
Engine compression ratio
- how does it affect performance and economy?
In
an internal combustion engine, a piston compresses a large volume of a
mixture of fuel and air into a very small space. The ratio of the
maximum piston volume to the minimum compressed volume is called the
"compression ratio."
Compressing
the fuel and air will make them burn faster, which (though I'm not sure
directly how) makes the engine run better. Due to the high compression
ratio (12.51) of the 11,000 RPM Hayabusa engine and the low compression
ratio (9.8:1) of the 6500rpm Mustang V8, I'm guessing that this allows
for a much higher redline - the faster burn speed of the compressed
fuel-air mix in the Hayabusa engine would allow it to complete burning
before the piston had completed its stroke at high RPMs.
There
are secondary benefits to high compression ratios, too. High
compression ratio engines burn both much more cleanly and much more
efficiently than lower-compression engines. For example, a diesel
engine, which burns fuel very differently to a gasoline engine, will
often give fuel economy 60% greater than its gas equivalent, even
though diesel only has about 10% more energy per gallon.
According
to Wikipedia, the increase in efficiency is due to the additional heat
and brownian motion caused by compression fully vaporizing the fuel,
which I think sounds a little fishy considering how much work is put
into cooling the fuel-air mix in turbocharged cars. Most other websites
say that it's due to the Carnot cycle, which I honestly do not
understand - could someone explain it?
Another
issue is engine efficiency as a function of RPMs. An engine limits
power by reducing the intake of fuel and air to an engine; if only half
the fuel and air is entering a piston, the compression ratio is
effectively halved as well.
Considering
all the advantages of high compression, one might wonder why anyone
would not use a high compression ratio. The answer is simple: The
increased heat density of the compressed gas will cause the fuel to
begin combustion without ignition by the spark plug, resulting in an
undesirable burn pattern. This detonation, or "knock", is often heard
as a pinging noise and can cause severe damage to your engine.
The
measure of a fuel's minimum ignition temperature and resistance to
detonation is its octane, which is not, as is commonly understood, a
measure of it's energy per liter. Before the advent of the catalytic
cracker, fuel was often below seventy octane, and engine compression
ratios were low - a Model T had a compression ratio of 4.5 to 1.
However, by splitting large molecules into smaller ones (cracking),
modern engines are both more efficient and better performing than their
older brethren.
Modern
gas has an octane of about 93 for premium in the US, and about 97 in
the rest of the world. 100+ octane gas can be had, but it's very
expensive (well over $5/gallon) and often has octane-boosting additives
which contain lead. However, all of these are dwarfed by ethanol, which
as an octane rating of a whopping 129. As a result, it can easily be
used in engines with a compression ratio of 15:1 or greater, and
despite having an energy density only 2/3 that of gasoline, it should
produce similar fuel economy in such an ethanol-optimized engines along
with very, very high redlines.
Running
higher compression ratios is a very hotly debated subject at the moment
and the two camps are extremely divided. As a lot of you probably know,
the two “camps” consist of the old school Cosworth era tuners who have
been building engines with lowered ratio’s for years (and have worked
well) and the newer breed of tuners (mainly stemming from USDM Honda
scene) who have had great practical and proven success building higher
compression turbo engines.
Let’s start with the
correct turbo choice for your application.
People
run smaller turbo’s because they want them to spool early and to
deliver a wider range of power. Turbo A (smaller turbo) running 1.0bar
will spool faster than Turbo B (slightly larger) running the same boost
pressure, but the amount of air moved by Turbo B (slightly larger) will
be greater at the given boost pressure as it’s moving more air? Thus
creating more power.
The
problem is that the more boost pressure you run, the more the charge is
heated by the turbo. This results in the temperature of the air/fuel
mix entering the combustion chamber to be significantly higher. Once
the piston is on it’s up stroke, this air and fuel mix is compressed
and something called adiabatic heating occurs. (*which is the increase
in temperature of a fluid when under pressure) if this air fuel mix
reaches the auto-ignition point of gasoline you get premature
detonation or det (The air/fuel mix auto igniting before the spark plug
fires – not like premature ejaculation).
By
upping the compression ratio in an engine, your increasing the heat
that is generated when the gas is compressed on the upstroke (but also
increasing the density of the gas/air mix and extracting more
mechanical energy) this means that your inlet temperatures and fuel
octane are significantly more important. Higher octane fuel has a
higher auto ignition temperature and is more difficult to burn (the
opposite to common belief that high octane fuel is actually more easily
ignited) thus making your combustion process more det resistant.
The
type of piston and shape of the combustion chamber, determines the
speed of the flame front that travels across the compressed mixture.
In
simple terms a higher compression ratio DOES create more power off
boost which gives you extra torque down the arse end. It’s really down
to static vs. effective compression.
Effective
compression is the sum of the static compression, plus the additional
compression added to the cylinder by a turbo or super charger, or any
other forced induction tool for that matter. Effective compression is
defined by the following formula:
E = C((B / 14.7) + 1)
Where E= Effective Compression, B= boost
psi, and C= Static compression. Also remember that 14.7 is equal to 1
bar of boost.
Let’s do an example. Let’s say we have a SR20DE bone stock with 10.4:1
static compression and slapped on a turbo kit. It’s now boosting 7psi.
That takes care of our variables. Let’s do the math.
E = C((B / 14.7) + 1)
E = 10.4((7 / 14.7) + 1)
E = 10.4((.476xx) + 1)
E = 10.4(1.476xx)
E = 15.35xxx
As
you can see, we come up with an effective compression ratio of 15.3 or
so. A motor in this effective compression range is easily daily driven
with proper fuel and timing adjustments/upgrades.
(Running on the same turbo back
to back)
If you’re running a 9.0:1 compression
ratio @ 1 bar you’ll achieve an effective compression ratio of 18:1
If you’re running a 7.5:1 static
compression ratio and 1 bar of boost you achieve an effective
compression ratio of 15:1.
For
the 7.5:1 to reach the same effective compression ratio you need to run
.4bar or 5psi more boost. Combined with the fact that you have less
“grunt” outside the boost threshold. Less grunt/torque means your
engine produces LESS of a bang when the combustion mixture ignites,
along with a slower burn due to a less compressed cooler mixture.
So
in a nutshell – if you’re running 2.0 bar of boost on a 7.5:1 static
ratio your achieving an effective compression ratio of 22.5:1 if you
run the same setup with 9.0:1 static ratio you get the same effective
compression ratio but with only 1.5 bar of boost and much better
drivability outside of the boost threshold and a better spool due as a
result.
Of
Course, if the compression ratio is too high then the adiabatic effect
will cause the mixture to auto ignite – so there is a line to be drawn
obviously.
When
building turbo engines static compression ratio is actually a bit of a
clumsy way of measuring what’s going on because your measuring the C/R
at atmospheric pressure not the desired. With that said, a higher C/R
engine will produce more power off boost and subsequently spool your
turbo slightly faster. You need to aim for a power goal (whatever that
is) and spec your turbo setup accordingly to produce the required air
at a reasonable pressure & temperature.
ALCOHOL FUELS
RIGHT
at this point it might be as well to point out to readers that the
handling of alcohol fuel, even in small quantities, is dangerous since
poisonous Methyl Alcohol is the basis of most of these fuels.
In
some cases to prevent it being used for drinking an additive is used,
called Pyridine, about one half per cent being the amount.
This gives it a nasty smell and a vile
taste, but pure fuel is, of course, without this deterrent.
The
problem still remains, however, since it can get into the system by
absorption through the skin or cuts, and can be inhaled from exhaust
gases.
The effects are cumulative and if enough
build up is allowed it oxidizes forming Formaldehyde causing blindness
and insanity.
The
use of rubber gloves, avoiding splashing and handling in confined space
and in general treating with commonsense, however, reduces the risks to
acceptable proportions.
Should, however, any get in the eyes
immediate medical attention is necessary.
For
those who have not handled alcohol fuel it might be as well to say it
is a clear, colorless liquid, cool in touch, with an odor different
from petrol, and has an attraction to moisture in the atmosphere.
ADVANTAGES AND
DISADVANTAGES
Let
us now investigate the advantages and disadvantages of going over to
this fuel, and at all times taking petrol as our reference level,
having in mind the basic requirements of fuel in the heat engine.
The
first question must be is it easy to obtain and the answer is there are
a number of garages retailing the fuel, in certain cases with other
fuels added in specified quantities.
Having obtained the fuel, as already
explained, it must be handled with care and commonsense.
There
is no real problem in keeping in store any quantity left over from one
meeting to another, provided it is kept in a can, or tank for that
matter, with the cap kept on during the store period, which can extend
into years, contrary to popular belief.
COST
Cost
of the alcohol depends on what other fuels have been incorporated, but
as guide pure alcohol is, in small quantities, about just over half as
much again as the cost of top grade petrol. You must bear in mind at
this point, however, you will require double the amount of alcohol as
compared to petrol for reasons which will be explained later.
Another
point to consider is that alcohol is a solvent and so far as certain
paints are concerned it acts as a perfect paint stripper. Alcohol also
has a very thorough scouring effect on tanks, pipe lines and so on, not
forgetting it can on certain
types of fiberglass tanks cause them to
disintegrate into a rather nasty sticky mess.
CONSUMPTION
Consumption
of alcohol will be, in rough figures, double that of petrol, due to the
calorific value being about half that of petrol.
The
correct air-fuel ratio for petrol is 14.1 to 15.1, but for alcohol it
is 7.1 to 9.1 so that means we must pass at least twice the weight of
fuel, in the case of alcohol, to heat the same amount of air to the
same temperature as we need for petrol.
Since
the specific gravity of the two fuels is near enough the same it means
in effect we have to pass through the jets double the quantity of the
fuel.
Apart from doubling up the flow capacity
of the jets, and we would add here that this does not mean doubling up
the diameter of
the jet hole as many people think, but, in fact, increasing the
diameter by 1.4 times or if you like by 40 per cent since a little
thought will remind you of the fact you are dealing with the area of
the hole in the jet and not the diameter.
It
is of little use increasing the capacity of the jet to pass double the
amount of fuel unless steps have been taken to establish that the fuel
lines, taps, float chambers and so on are also capable of passing
double the fuel and the actual flow should be measured.
RICH SIDE
Now
unlike petrol you will find alcohol fuel will continue to provide
increased power for a mixture well above the ideal mixture strength and
you can always tend, therefore, to jet up on the rich side, and so
avoid any possible chance of running into troubles through weak mixture
causing burnt valves and holed pistons.
This
larger amount of fuel compared to petrol and especially as it is a fuel
with much higher latent heat value tends to do two things. The density
of the charge entering the engine is higher than petrol and a greater
weight of mixture is therefore being exploded.
This
is a fuel with a large cooling effect provided by part of it
evaporating after it has reached the combustion chamber and so tending
to cool the valves, piston and so on.
Some
may well get into the combustion chamber as liquid, due to the
reduction in temperature of the induction system, pipes, carburetor,
etc., and so extending the cooling effect, in the process counteracting
the effect of the high internal temperature.
In
view of this amount of fuel entering the chamber, with possibly some of
it in liquid form, the ignition system must be beyond reproach since if
the spark is weak the mass of fuel will just soak the plug and then at
once ignition troubles arise affecting starting in particular.
Owing
to the use of alcohol a higher compression ratio can be used with this
fuel as compared with petrol, another consideration is the type of plug
used which will be a hotter type than used before with petrol.
NINETEEN TO ONE
We
have just mentioned the higher possible compression ratio used with
alcohol and the limit that can be used with any particular fuel depends
on the tendency of the fuel to detonate.
As
a rough guide the ratio for petrol is limited to about ten to one, or
with certain additives to as much as 12 to one. With alcohol, however,
you can go up to 19 to one or higher in certain cases. (For all
practical purposes however, 14 to one should be considered the maximum
usable ratio in modern short stroke automotive engines.)
The
possible use of a much higher ratio, of course, means we get a higher
power output from the engine, and this, in fact, is almost the main
advantage of alcohol fuel.
DETONATION
Detonation
with alcohol fuel is really not a problem, but pre-ignition is, or
could be unless the mixture is kept well on the rich side.
The
reason for this is that if the mixture is on the weak side it burns
slowly and can still be so doing when the exhaust valve has opened
which then becomes overheated. This in turn ignites the next charge
before the correct time, the whole process becoming a chain reaction
causing even more rise in temperature and so it goes on until the
piston holes and other damage then follows.
The
first signs of this process taking place are a loss of power, a general
rise quite quickly of overall temperature, the head in particular.
To avoid this, run on the rich side
always and use plugs with a good heat capacity.
It
might be worth mentioning at this point that an engine set up correctly
for running on alcohol, even though on a rich mixture, will be found to
be (compared to petrol), a much cleaner running engine inside the
cylinder head, and provided the ignition side is up to its job there
will be less fouling of plugs than on petrol.
IGNITION SETTING
Due to the different rate of burning of
alcohol compared to petrol the ignition setting will have to be changed.
It will have to be advanced and the
amount necessary will depend on the shape of the cylinder head and
general design.
For
example, on a well designed hemi-head an extra five to six degrees
might well be enough, whereas on a poor designed head it might be
something like 15 degrees.
Optimum
ignition setting is tied up with the air-fuel ratio and it will be
found that with alcohol it is not so critical as with petrol, that is
to say the drop off of power is not so progressive as will be seen
later.
STARTING
Provided
the engine is set up for running on alcohol correctly there should be
little trouble in starting except perhaps on a very cold day and it
should be possible to start up on the fuel mix used for the actual
racing.
The
main problem, due to the cooling effect of the fuel, is to get the
engine to operating temperature in the short time available from
fire-up to staging.
For this reason so far as motor cycle
type engines are concerned, you will note,
in
many cases, the finning on the cylinder barrels and heads is almost
eliminated. This, by the way, also helps to increase the power to
weight ratio, or if you like tends to counteract the weight of the
extra amount of fuel carried as compared to petrol.
LIMIT
From
reading this far, you should have come to the conclusion that if your
engine is now on its limit running on petrol, while large increases of
power are obtainable by the use of higher compression ratios it is
possible to get a reasonable increase in power output, ten per cent or
so, with the existing ratio, provided you make quite certain you get
enough fuel through to the engine and, in fact, that you tend to run on
the rich side.
Once
you have gone over to alcohol and obtained satisfactory running, you
have commenced an extension of your power output by anything up to 25
per cent as you adapt the engine to run with the new fuel.
The
rather attractive feature that you tend, even with the increase of
power to stand less chance of doing damage to the engine than when on
petrol should also be considered.
FINAL POINT
One
final point to consider. If you change over to alcohol from petrol
where you were using a mineral oil, it is not necessary to change over
to a castor based oil. For modern engines, the present type additive
mineral oils offer a higher performance level than the additive castor
based oils, and under controlled conditions the light viscosity oils
have an advantage where the warm up time is limited.
BASIC FUEL CHARACTERISTICS
|
GENERAL
DESCRIPTION
METHANOL (Methyl Alcohol)
CH30H is a volatile, highly inflammable, water-clear liquid with a
mildly spirituous odor Miscible with water or nitromethane in all
proportions and almost all with petrol.
|
BASIC
CHARACTERISTICS
|
Flash Point
|
Boiling Point
|
Freezing Point
|
Specific Gravity
|
Lbs/Gall approx
|
|
F C
|
F C
|
F C
|
|
|
61 16 148 64 -144 -97 0.796 8
|
NITROMETHANE
CH3NO2
is an inflammable water-clear liquid with a mild odor, containing
approximately 53% by weight of oxygen. Water will mix with nitromethane
to the extent of 2.5% only, by volume. |
110 43 214 101 -20 -29 1.13 11.25
|
ACETONE (Dimethyl
Ketone) CH3COCH3
is a highly volatile, highly inflammable, water-clear liquid with a
strong, sharp, characteristic odor. Miscible with all the chemicals
listed here, and water. |
0 -18 133 56 -138 -94 0.791 8
|
ETHER
(Diethyl Ether) C2H5OC2H5
is an extremely volatile, highly inflammable, water clear liquid with a
strong, lingering, characteristic odor. Miscible with all the chemicals
listed here but not with water. |
-40 -40 95 35 -183 -116 0.714 7
|
BENZOLE,
(Benzene) C6H6
is an inflammable water-clear liquid with a dull sweet odor Miscible in
most proportions with all the chemicals listed here but not with water. |
12 -11 176 80 41 5 0.879 8.75
|
NITROBENZENE
C6H5NO2
is an inflammable, yellow, oily liquid with a strong odor of almonds.
Miscible in most proportions with all the chemicals listed here but not
with water. |
190 88 412 211 41 5 1.20 12
|
PROPYLENE
OXIDE (1 :2. Epoxypropane) CH3-CH-CH2
is an extremely volatile, very reactive, highly inflammable,
water-clear liquid with a light gaseous odor. Miscible with all the
chemicals listed here but only partially with water. |
32 0 93 34 -155 -104 0.83 8.25
|
UCON
LB625 (Polyalkalene glycol)
A water-clear synthetic lubricating oil with exceptionally high film
strength properties. Miscible with all the chemicals listed here but
not with water. |
430 221 - - -25 - 32 1.0 10
|
|
|
|
Conservative
Maximum Compression Ratio
|
Air/Fuel
Ratio for Max Power lbs/lbs
|
Energy from
Combustion B.T.U/lb
|
Cooling
Effect (Latent heat of Vaporization) B.T.U./lb
|
|
Use in Internal
Combustion Engines
|
|
Methanol
|
|
Methanol
permits the use of very high compression ratios when unsupercharged or
high boost pressures when supercharged. The large cooling effect
increases volumetric efficiency and is of particular use in the
supercharged engine reducing charge temperature after compression. A
tendency to pre-ignition is most noticeable at weak mixture levels. |
| Nitromethane |
|
6.5 : 1
(10 : 1 with rich mixtures)
|
2.5 : 1 to
0.5:1 at least
|
5000
|
258
|
|
Nitromethane
enables considerable power increases to be obtained (70 percent minimum
with proper use). Most often used blended with methanol, in various
propor ,tions to provide power increases consistent with engine
strength, etc. A tendency to detonation is reduced by an increase in
mixture strength, reduction in engine temperature, reduction in
compression ratio or the addition of methanol. |
|
Acetone
|
17 : 1 9.4 : 1 12,500 225
approx
|
As
a basic fuel acetone appears to have all the required characteristics,
these in general Iying midway between methanol and petroleum. An
exception is its very high anti-knock rating which approaches that of
methanol. Other uses are as an additive to other fuels, notably to
methanol to reduce pre-ignition sensitivity and promote easier starting
under low temperature conditions, up to 10 percent for this purpose. |
|
Ether
|
|
Not
used as a basic fuel in spark ignition engines due to its very low
knock-rating, but this characteristic is desirable in the small
high-speed diesel engine where it is used in relatively large
percentages (approx. 15 percent to 35 percent by volume) as an
additive. Its volatile nature and low flash point make it useful as an
additive tuP to 5 percent) to improve starting and give a rapid
throttle response. |
|
Benzole
|
15 : 1 10.8 : 1 17,300 153
|
Most
often used blended with methanol to give a greater energy per unit
volume with reduction in the latent heat vaporization, this being a
compromise often used for long distance racing where fuels other than
petrol are allowed. |
|
Nitrobenzene
|
not known 8 : 1 10,800 143
|
Blended
in very small proportions with other fuels it is thought to act as an
ignition accelerator. As this material has a strong odor even after
combustion it is sometimes used as an additive in other fuels (approx.
0.5 percent) to mask the normal exhaust odor making it difficult to
detect the base fuel type. |
|
Propylene Oxide
|
not known 9.6 : 1 14,000 220
|
Used
as an ignition accelerator additive particularly with nitromethane (up
to 20 percent by volume with pure nitromethane) where noticeable
increases in power are possible. Easier starting and smoother running
are other benefits when blended with most other fuels (up to 5 percent) |
|
Ucon
|
At 0 F this oil
compares to SAE 20 at the same temperature, and at 210 F it compares to
SAE 50 at the same temperature |
Used
to advantage in all two stroke engines operating on fuel/oil mixtures.
The unusually high him strength properties allowing a reduction in the
amount of oil in the fuel by as much as 55 percent. Of particular use
in very small high speed two stroke engines where the normal oil
content can be up to 30 percent of the total volume, with the attendant
restriction on the amount oF fuel that can be burnt. |
NOTES
|
Methanol
|
Corrosion
- Magnesium: Attacked.
- Tin: White deposit (long term).
- Polythene: Cracks (long term).
- Paints: Most attacked severely.
- Perspex: Attacked.
|
Handling
Poisonous; do not allow to come
into contact with skin as repeated absorption may have long term
effects on health.
|
| Nitromethane |
- Copper/Alloys: May be attacked.
- Polythene: Generally resistant.
- Paints: Most attacked severely.
- Perspex: Attacked.
|
Do not allow
to come into contact with caustic soda, amines or hydrazine. Pipeline
pressures must be kept below 100-lb/sqlin. |
|
Acetone
|
- Metals: Resistant.
- Polythene: Cracks (long term).
- Paints: Most attacked severely.
- Perspex: Attacked.
- Neoprene: Some attack.
|
Low flash
point presents considerable fire risk. Extinguish with dry powder or
CO2. |
|
Ether
|
- Metals: Resistant.
- Polythene: Cracks (long term).
- Paints: Most attacked severely.
- Perspex: Attacked.
- Neoprene: Some attack.
|
Very low flash
point presents serious fire and explosion risks. Vapor is heavier. than
air and anesthetic. |
|
Benzole
|
- Metals: Resistant.
- Polythene: Generally resistant.
- Paints: Some attacked.
- Perspex: Some attack.
|
Poisonous;
strong vapors must not be inhaled, may affect blood tissues permanently. |
|
Nitrobenzene
|
As for benzole
|
Very
poisonous; do not allow to come into contact with skin or inhale vapors. |
|
Propylene Oxide
|
- Metals: Most resistant.
- Polythene: Cracks (long term).
- Paints: Most attacked severely.
- Perspex: Attacked.
- Neoprene: Some attack.
|
A very
reactive chemical, must not be allowed to come into contact with
copper/alloys or rust, reaction may be violent. |
|
Ucon
|
No problems
|
No problems
|
Alcohol Problems
For the racer there seem to be many positives for using alcohol as a
fuel; are there any downsides? Yes, there are a number of issues that
alcohol brings to the party that are not even considerations with
gasoline fuels. The first is that alcohol is hygroscopic. It will
absorb water out of the air if it’s exposed to the environment. This
little feature can make a perfectly acceptable jug of fuel not worth
using if the water content gets too high. This feature of alky fuels
is, and has been, the bane of many tuners as they make changes to the
fuel system only to find that the fuel was contaminated with water.
This
is also a real problem in areas that have a good bit of humidity in the
air. In the Southwest it’s not a big issue but it still means that any
alcohol that is stored needs to be in containers that are not vented
and that the fuel should not be exposed to the environment any longer
than possible.
Another downside is that many
of the rubber type seals that are used in gasoline fueled cars don’t
hold up when the fuel is changed to alcohol. They don’t react well with
alcohol fuels, often degrading and no longer offering an acceptable
seal, or even worse they degrade and contaminate the fuel downstream of
their location. While this seems like a real issue, it’s simply
rectified, by using seal materials that are resistant to alcohols, from
the tank to the end of the fuel delivery system.
Chemistry 101
The
chemical makeup of alcohol is very corrosive to many of the coatings
that are typically used on metals in the fuel system. It’s not uncommon
for metal components to get surface oxidization and pitting as a result
of alcohol fuels. This becomes a real issue if the alcohol is allowed
to sit in the fuel system between races. The fuel system should be
maintained between races to prevent the alcohol in the system from
turning into what is a very strong corrosive agent.
If
the fuel system isn’t cleaned frequently, preferably after each race
day, the corrosive nature of alcohol will play havoc with the metal and
rubber components in the fuel system, especially those components not
designed for this type of fuel. This isn’t a real issue as most racers
who are using alcohol fuels are already familiar with the required
maintenance. For those not familiar with the maintenance rigors
required when using alcohol fuels; education comes quickly and with a
vengeance.
Butanol
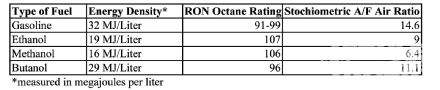
has some unique characteristics; it’s the one alcohol that most closely
mimics gasoline from an energy density perspective. Its stochiometric
air/fuel ratio is the closest to gasoline. Due to its chemical makeup,
butanol isn’t as corrosive as methanol or ethanol. While all of this
sounds great, there are some issues that prevent butanol from being a
viable racing fuel at this point in time. First, is that it has a
fairly high melting point and at cooler room temperatures more closely
resembles Vaseline than a liquid fuel. However, it does mix well with
gasoline and that has some real positives for the passenger car world;
however it’s not a real boon to the racing world, yet. At this time we
will still focus our attention on methanol, while ethanol is gaining
more acceptance.
Failure to properly
maintain an alcohol fuel system will result in, aside from the
corrosion, a grit like substance, almost a fine sand type of residue,
in the lines and around aluminum parts. This grit is the result of an
increased electrical conductivity that alcohol has over gasoline fuels.
The grit is from the galvanic corrosion caused by the greater
electrical conductivity from the fuel as it interacts with the various
different metals in the fuel system. This contamination will migrate
throughout the system clogging fuel filters, fuel jets, and generally
cause havoc within the fuel system.
It’s
often thought that alcohol makes power because it has a greater amount
of energy. This isn’t exactly true; in fact, the type of alcohols that
are commonly used in racing have less heat energy than gasoline based
on the volume. There are, in fact, four types of alcohols of which only
methanol and ethanol are currently used as fuels in the racing world.
The other two types of alcohols, propanol and butanol, aren’t used
commonly used. Propanol has more uses as an industrial solvent than as
a fuel while Butanol is an interesting chemical.
More Power
So,
just why does alcohol make more power than gasoline if it has less
energy per pound than gasoline? Good question! Obviously, you will have
to run more of the alcohol-based fuels to get the same power, how much
more will depend on the type of alcohol you’re running. With methanol
and ethanol it’s about 40 percent more than gasoline. Let me espouse
some of the good characteristics that alcohol brings to the table.
First,
when you burn alcohol one of the byproducts of combustion is oxygen.
This helps enhance the combustion process. Another is the cooling
effect of alcohol as it “vaporizes” in the inlet track. This helps
create denser air as the air/fuel charge enters the engine, another
positive. The cooling effect also helps to cool the engine, at least on
the inlet side of the equation. Remember, producing horsepower is all
about creating and controlling heat.
Another
positive feature about alcohol that is seldom discussed is that the
incoming fuel charge, the mixture of air and alcohol, is easier to
compress than a mixture of gasoline and air. The alcohol doesn’t
vaporize as well or as completely as gasoline as it comes out of the
carburetor or the injector. While gasoline forms a more complete vapor,
alcohol forms a “vapor” made up of many very small droplets of fuel
suspended in the incoming air/fuel stream entering the engine. Then
during the compression stroke, the heat of simply compressing the
incoming air/fuel mixture completes the vaporization process.
So,
from a mechanical perspective, your engine uses a smaller percentage of
the power it’s making to sustain continued operation. Long story short,
an alcohol mixture takes less energy to compress than a gasoline
mixture. And, as an added bonus the last vaporization step also helps
to further cool the mixture. Remember, cool, in this case, is a
relative term as compared to a gasoline mixture.
Additionally,
an engine that is burning methanol or ethanol can support a much higher
compression ratio. It’s not uncommon to see alcohol engines using as
high as 13:1 or 14:1 compression rations with little fuel-related
problems. Of course, high compression engines have other mechanical
issues that aren’t related to fuel. That said, alcohol can support some
very high compression engines without the fuel causing detonation
issues which can occur if the wrong grade of gasoline is used.
|